Involves the Removal of Hydrogen Electrons and Co2
The CO2 molecule has two carbon-oxygen bonds OCO. During photosynthesis plants take in carbon dioxide CO2 and water H2O from the air and soil.
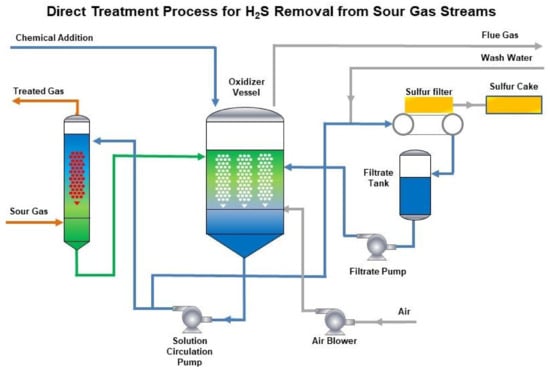
Catalysts Free Full Text Direct Selective Oxidation Of Hydrogen Sulfide Laboratory Pilot And Industrial Tests Html
Involves the removal of hydrogen electrons and CO2 from the substrate molecule.

. The electrons are unequally shared with the oxygen atom spending extra time with electrons than the hydrogen atoms. Involves reduction of NAD. A positive shift in band gap energy from 315 to 279 eV was achieved by incorporating 8 wt g-C 3 N 4 on TiO 2 surface.
Oxidation is a process which involves. Degrades glucose to CO2 and H2O. Involves the use of oxygen to pick up excess hydrogen and electrons.
Reduction can be considered all of the following. The rate of hydrogen production rate reaches 68 mmolg 1 h 1 for the heterojunction containing 8. Involves the use of oxygen to pick up excess hydrogen and electrons.
This can be achieved by reducing the oxide with coke carbon or magnesium metal. A water molecule abbreviated as H2O is an instance of a polar covalent bond. Electron transport chain Involves the removal of hydrogen electrons and CO2 from the substrate molecule.
Addition of oxygen removal of hydrogen addition of non-metal removal of metal Increase in ve valency loss of electrons and increase in oxidation number. Electron transport chain Krebs cycle Produces the most ATP Electron transport chain Involves the use of oxygen to pick up excess hydrogen and electrons. Electron transport chain Involves the removal of hydrogen electrons and CO2 from the substrate molecule.
Krebs cycle and electron transport chain. A sharing of a pair of electrons between a hydrogen nucleus and either and oxygen or nitrogen nucleus c. This transforms the water into oxygen and the carbon dioxide into glucose.
Breakdown of glycogen to release glucose. TAP THE ARROWS BELOW TO ADVANCE. Within the plant cell the water is oxidized meaning it loses electrons while the carbon dioxide is reduced meaning it gains electrons.
Degrades glucose to CO2 and H2O. Removal of CO2 is decarboxylation. When CO2 is heated with coke at about 800 degree C one carbon-oxygen bond is broken to form carbon monoxide CO.
Enzymes in matrix of mitochondrion remove H and CO2 from pyruvate. 92 of fluorescein dye was decomposed on nanocomposite with 8 wt g-C 3 N 4 compared with 45 dye removal on pristine TiO 2. Krebs cycle Glycolysis Glycolysis Occurs in the cytosol of a cell.
A sharing of a pair of electrons between a hydrogen nucleus and an oxygen nucleus b. During photosynthesis plants take in carbon dioxide CO2 and water H2O from the air and soil. These electrons and hydrogen atoms are used for the reduction of carbon dioxide.
2Mg O2 2MgO. Formed when an electronegative atom of a molecule weakly interacts with a hydrogen atom that is already. Involves the removal of hydrogen electrons and CO2 form the substrate molecule.
This transforms the water into oxygen and the carbon dioxide into glucoseOct 24 2019. A redox reaction is also called an oxidation-reduction reaction and involves a transfer of electrons between more than two species. That is each bond involves a pair of shared electrons formally with one electron from each of the atoms at.
As shown in Figure 2 Co-electrolysis has significant advantage over separate electrolysis of water and CO. I Addition of oxygen. The oxidation state of carbon decreases in this process.
Krebs cycle and ETC. Occurs in the cytosol of a cell. Krebs cycle and ETC.
Glycolysis Glucose serves as the initial reactant. Electron transport chain Involves the removal of. Hydrogen peroxide H2O2 has a structure HOOH where each dash in this structural formula is a single covalent bond.
The addition of hydrogen the removal of oxygen or the gain of electrons Electrolysis is generally considered to be the decomposition of a species when an electric current is passed. Krebs cycle Glycolysis Glycolysis Occurs in the cytosol of a cell. The process takes place at around 800C.
Since electrons spend extra time with the oxygen atom it carries a partial unfavorable cost. Breakdown of glycogen to release glucose. Within the plant cell the water is oxidized meaning it loses electrons while the carbon dioxide is reduced meaning it gains electrons.
A half reaction for an oxidation process always shows electrons on the reactant side of the half reaction. Krebs cycle and ETC. INVOLVES THE REMOVAL OF HYDROGEN ELECTRONS BY COENZYMES AND CO2 PRODUCTION FROM THE SUBSTRATE MOLECULE.
The product of oxidative decarboxylation of pyruvate is an acetyl group which is accepted by CoA. The electrons reduce carbon dioxide to form sugar molecules or glucose. The technology is a combined process that involves steam electrolysis CO2electrolysis and the reverse water gas shift RWGS reaction.
Removal of H is oxidation. You just studied 13 terms. During photosynthesis plants take in carbon dioxide CO2 and water H2O from the air and soil.
The formation of citric acid from oxaloacetic acid and an acetyl group begins. In industry hydrogen is often produced using natural gas which involves the removal of hydrogen from hydrocarbons at very high temperatures with about 95 of hydrogen production coming from steam reforming around year. Within the plant cell the water is oxidized meaning it loses electrons while the carbon dioxide is reduced meaning it gains electrons.
Occurs in the cytosol of a cell. Produces the most ATP. Ii Removal of hydrogen.
Hydrogen is versatile and can be utilized in various ways. Involves the removal of hydrogen. Electrons and CO2 from the substrate.
This transforms the water into oxygen and the carbon dioxide into glucose. 28A hydrogen bond is _____. Hydrogen is accepted by NAD.
So breaking a bond in CO2 involves removal of oxygen or reduction. Produces the most ATP. Electron transport chain Krebs cycle Produces the most ATP Electron transport chain Involves.

Catalysts Free Full Text Removal Of Hydrogen Sulfide From Various Industrial Gases A Review Of The Most Promising Adsorbing Materials Html

Removal Of Hydrogen And Co 2 From A Substrate Is Called
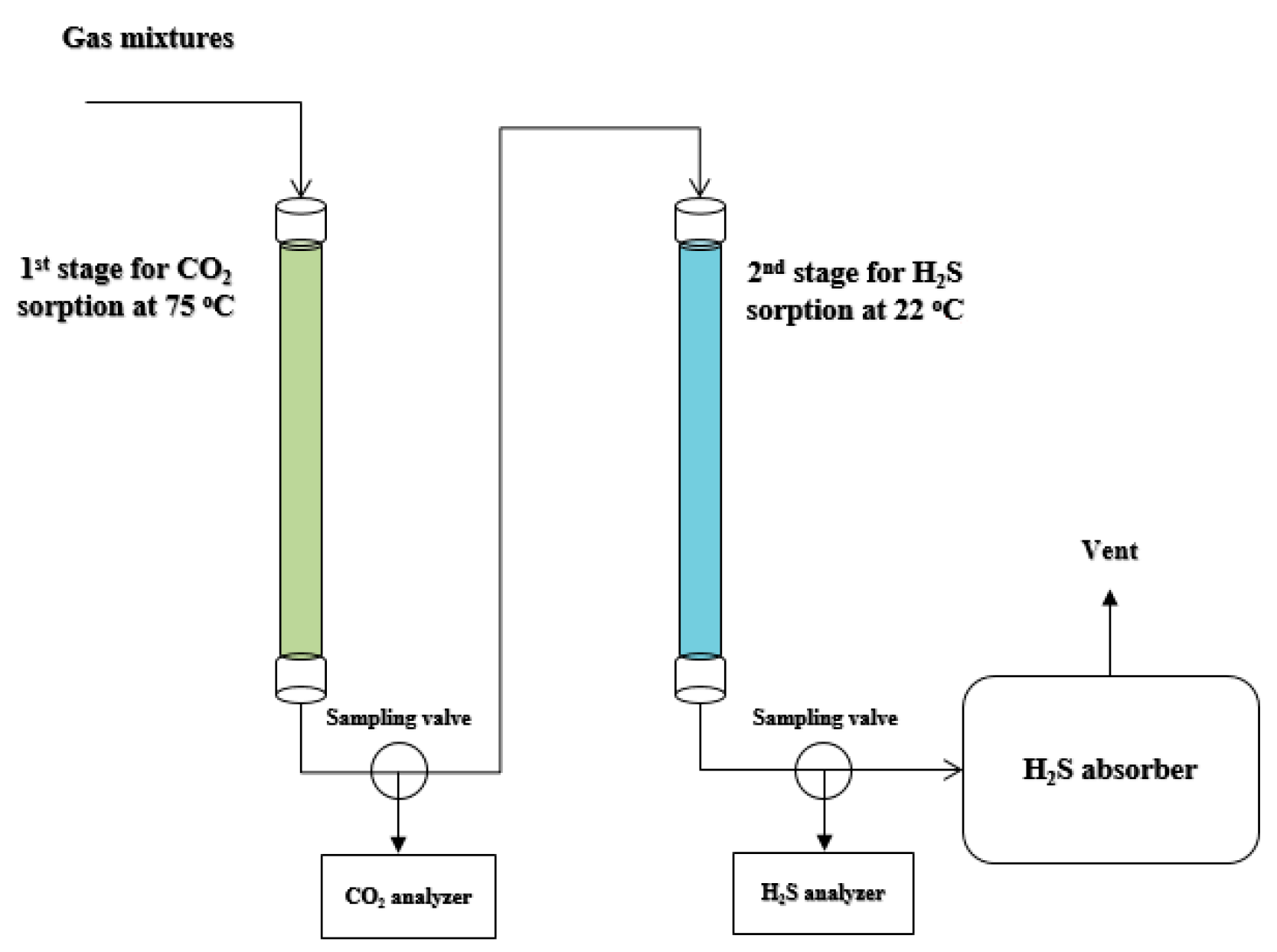
Catalysts Free Full Text Removal Of Hydrogen Sulfide From Various Industrial Gases A Review Of The Most Promising Adsorbing Materials Html

Perspectives On Reactive Separation And Removal Of Hydrogen Sulfide Sciencedirect
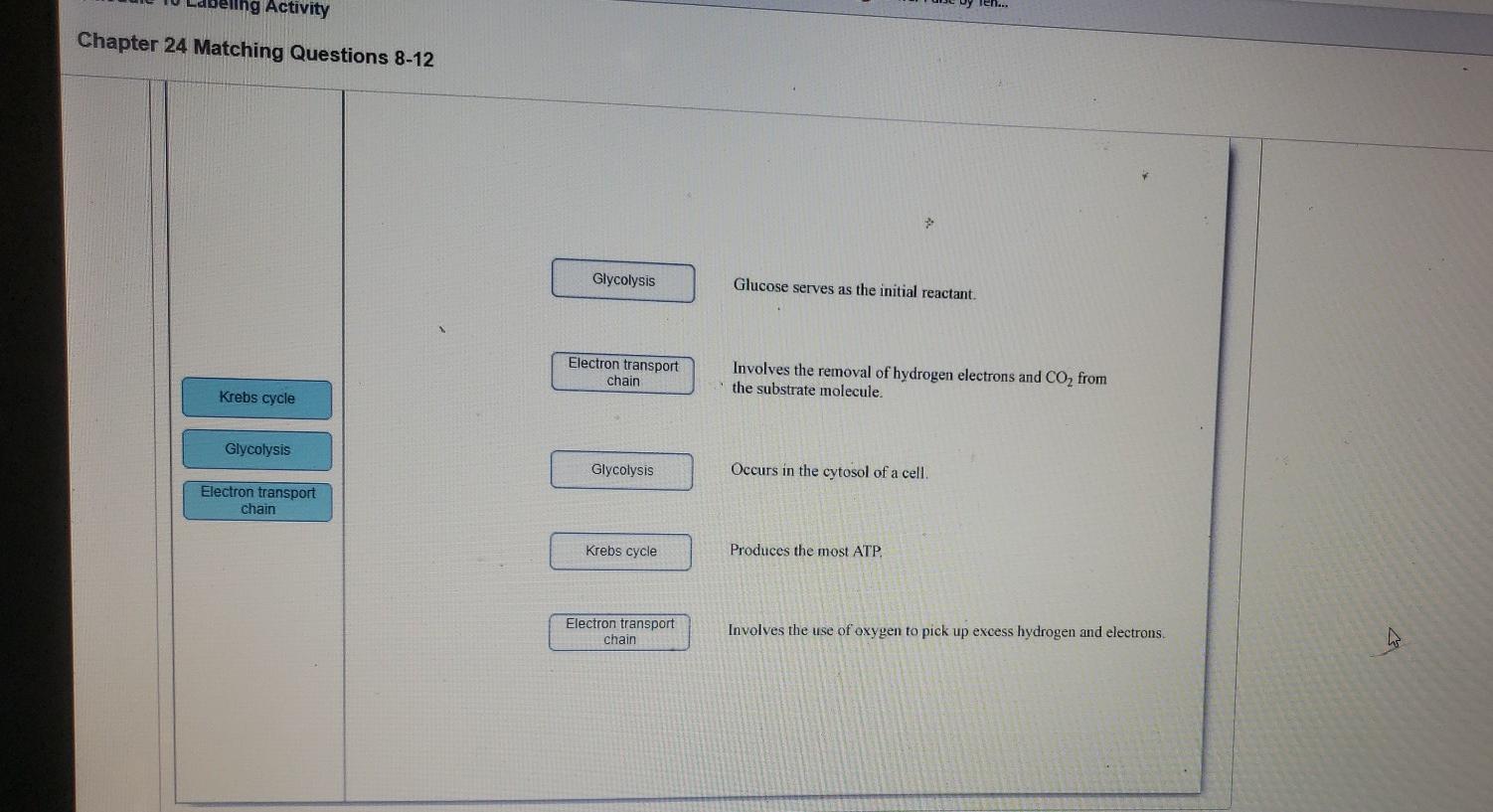
Solved Activity Chapter 24 Matching Questions 8 12 Chegg Com
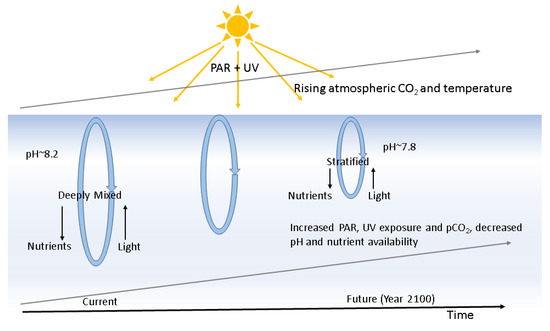
Sustainability Free Full Text Phytoplankton As Key Mediators Of The Biological Carbon Pump Their Responses To A Changing Climate Html

Anatomy And Physiology 2 Mastering A P Nutrition And Metabolism Flashcards Quizlet

Removal Of Hydrogen And Co 2 From A Substrate Is Called
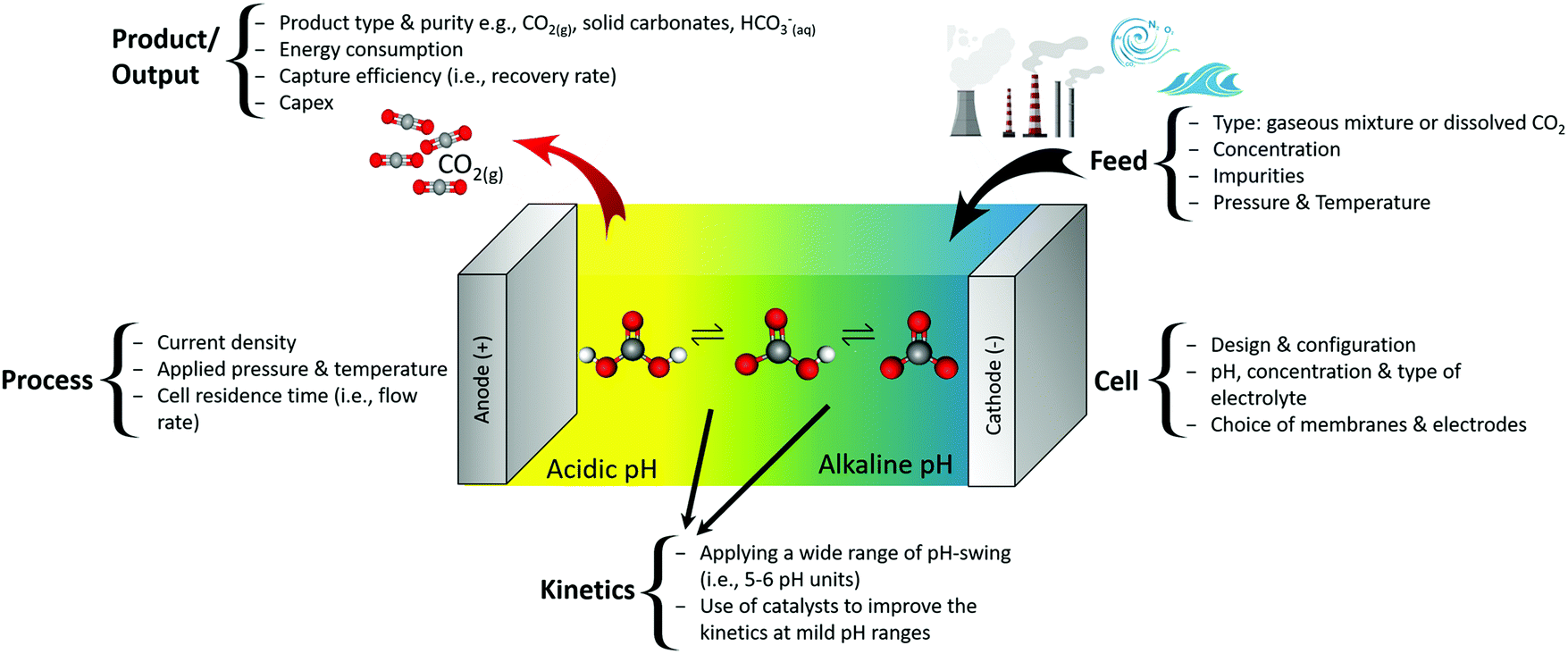
Electrochemical Carbon Dioxide Capture To Close The Carbon Cycle Energy Environmental Science Rsc Publishing Doi 10 1039 D0ee03382k
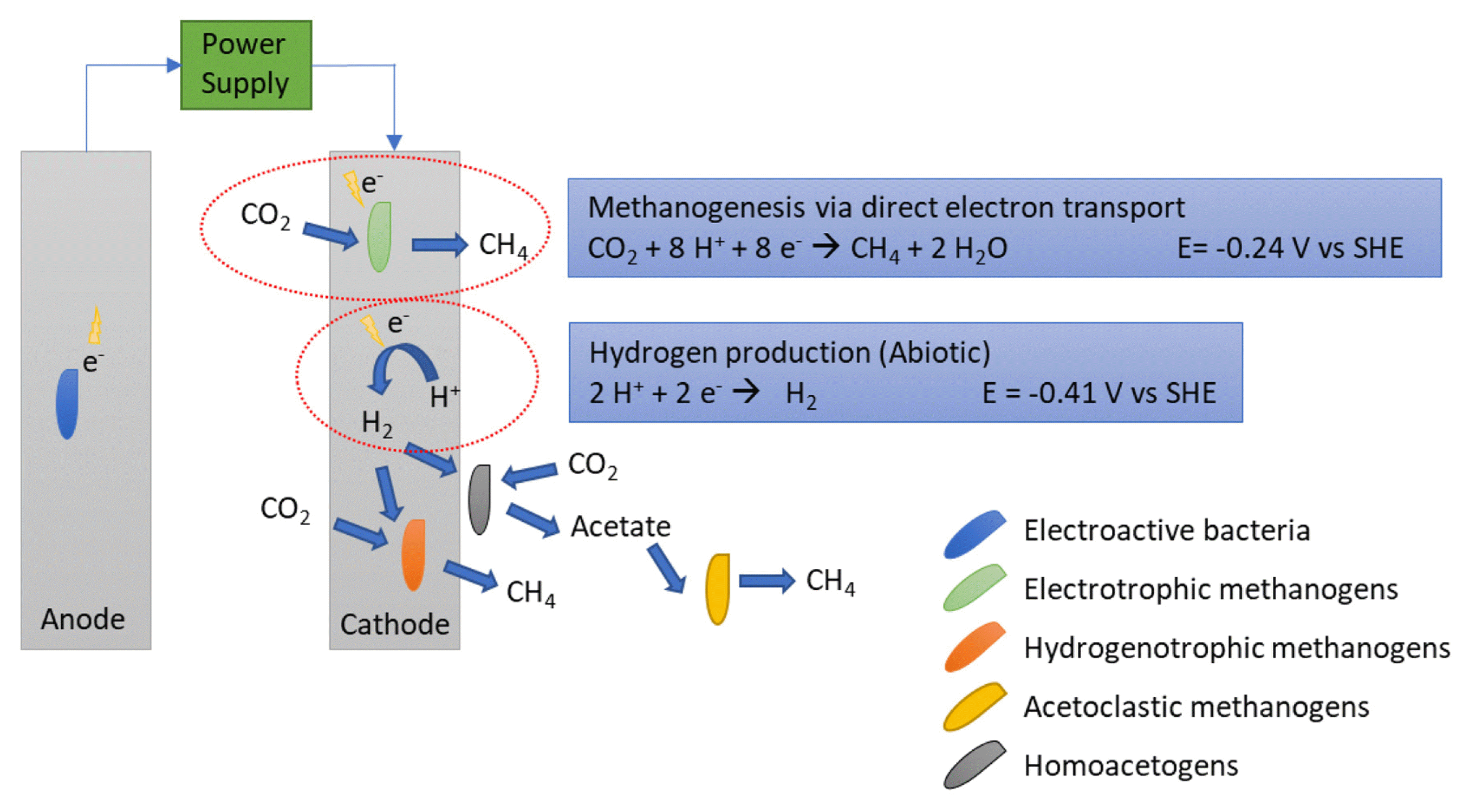
Microbial Electrolysis Cells For Electromethanogenesis Materials Configurations And Operations

Electron Transport Chain An Overview Sciencedirect Topics

Unit L Energy And Respiration Ppt Download
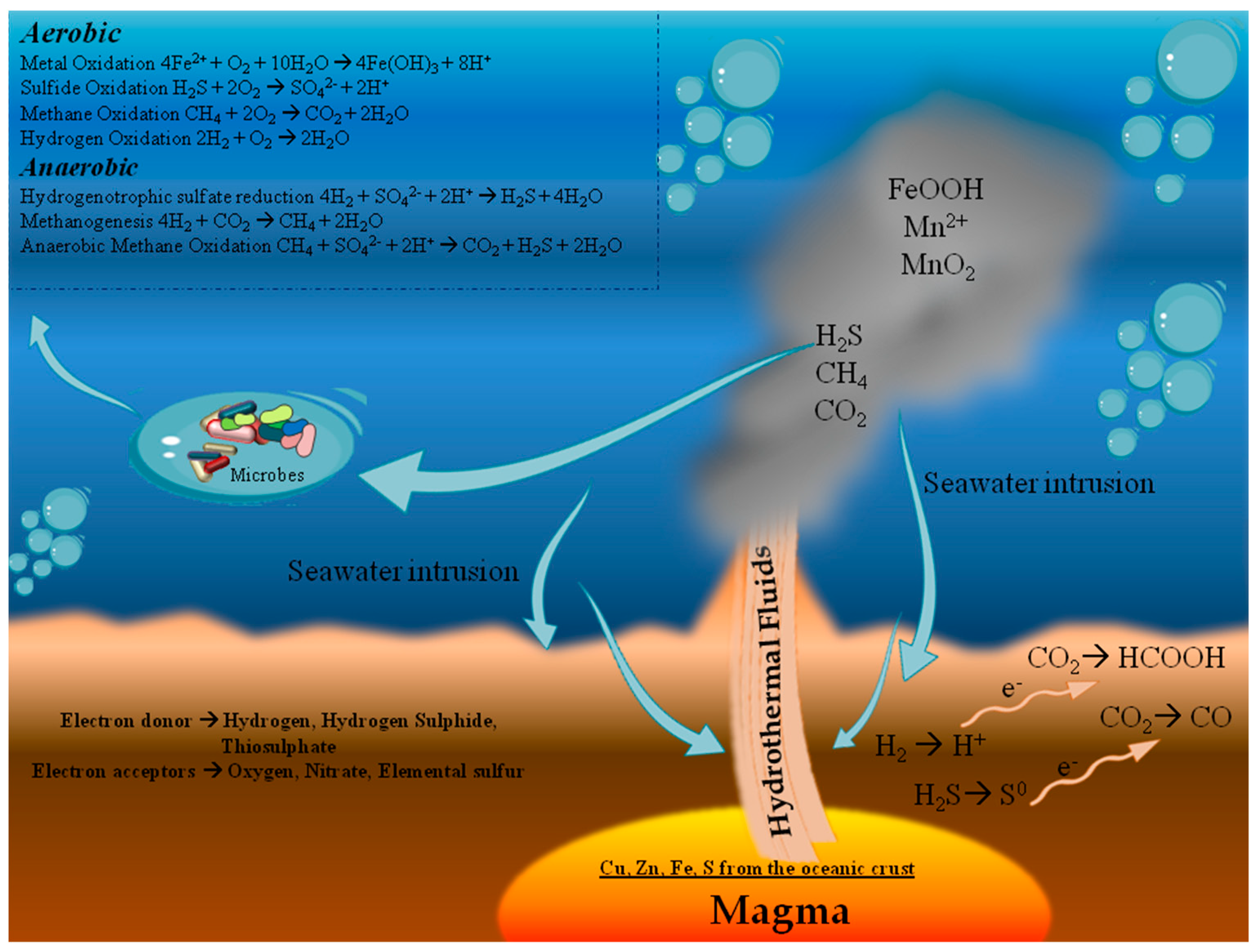
Minerals Free Full Text Ecological And Biotechnological Relevance Of Mediterranean Hydrothermal Vent Systems Html




Comments
Post a Comment